Pyranylidene/trifluoromethylbenzoic acid-based chromophores for dye-sensitized solar cells
Pyranylidene/trifluoromethylbenzoic acid-based chromophores for dye-sensitized solar cells
Raquel Royo, Amelia Domínguez-Celorrio, Santiago Franco, Raquel Andreu, Jesús Orduna
Dyes and Pigments 206 (2022) 110566
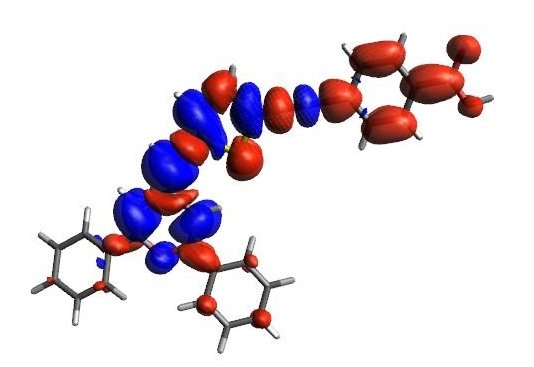
Abstract: The synthesis and photovoltaic study of five dyes based on 4H-pyranylidene moiety as donor moiety is reported. A thiophene unit conjugated with/without an ethynyl bond acts as the π-relay and benzoic acid as the anchor group with/without a trifluoromethyl group. Their electrochemical and optical properties are analyzed by using a joint experimental and theoretical approach. The presence of the trifluoromethyl group leads to an enhancement of the molar extinction coefficient, being slightly when a hexyl chain is introduced in the thiophene ring, but does not modify the oxidation potential.
For the preparation of derived solar cells an antiaggregant is essential in all cases. The photovoltaic performance is sensitive to the structural modification of the dye: the CF3 group and the hexyl chain of the thiophene spacer were shown to improve the efficiency. The lack of a triple bond in the π-spacer involves a lower photovoltaic efficiency, and the trifluoromethyl group leads to a lower dye-load, but a decrease of the recombination processes. These results are in accordance with the electrochemistry impedance spectroscopy studies carried out. Moreover, the organic dyes have been also tested with a fluorescent lamp (indoor conditions), leading to an increase of the efficiency, reaching a 36% for the best dye.