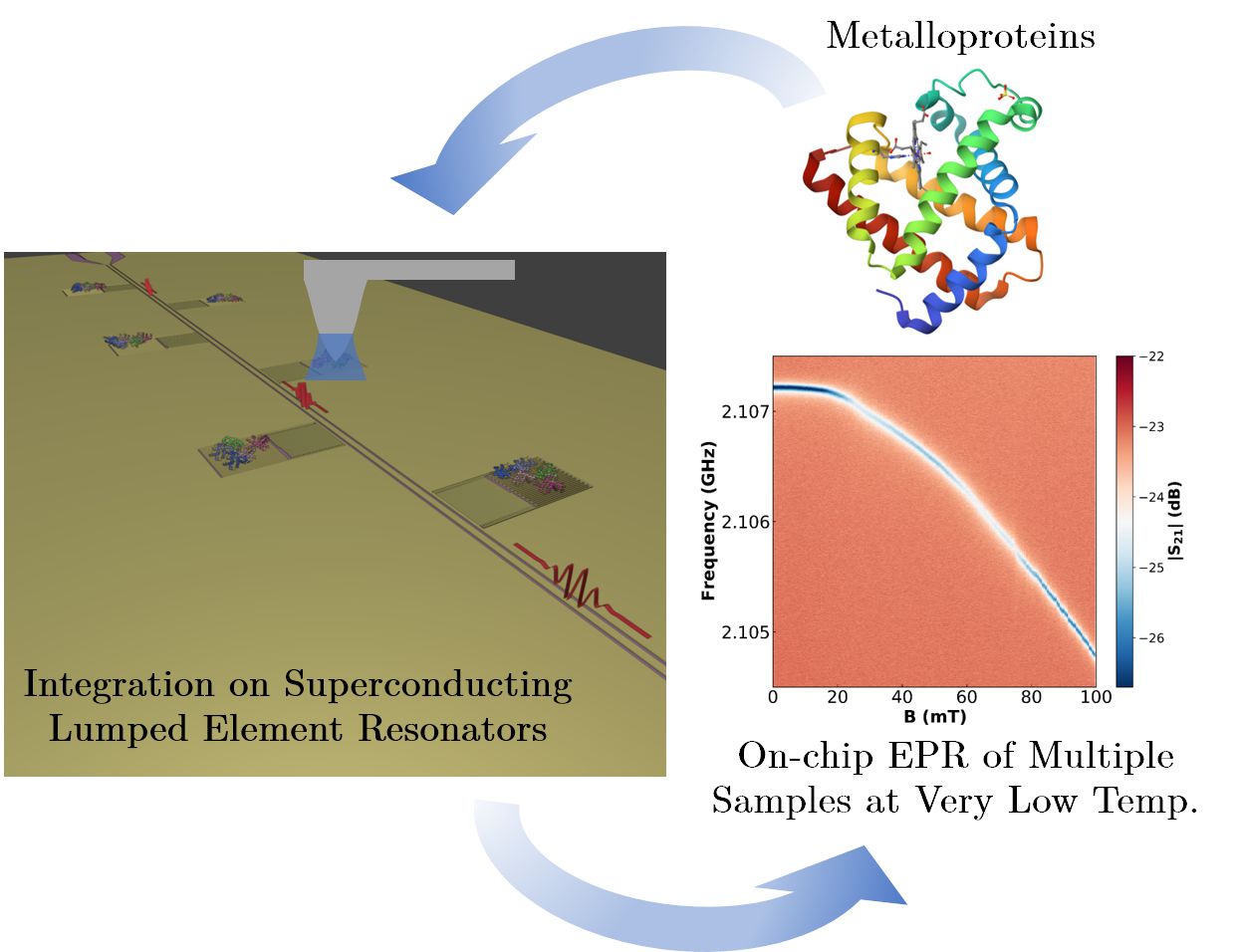
On-chip EPR spectrometry of metalloproteins using superconducting lumped element resonators
Researchers from the Quantum Materials and Devices | Q-MAD and HYMAT groups at INMA, in close collaboration with researchers from the Centre for Astrobiology (CAB, CSIC-INTA), have developed an EPR-on-chip platform based on superconducting LER resonators for the multiple characterisation of very small amounts of metalloproteins. The sensor has been verified by integrating controlled amounts of myoglobin molecules using dip-pen nanolithography (DPN). The average spin-photon coupling value is high, at around 9 Hz, suggesting that the DPN integration method generates a near-optimal interface between the molecules and the chip surface. The sensor combines an increase in measurement sensitivity of between 3 and 4 orders of magnitude compared to conventional EPR equipment with the ability to characterise multiple samples at once at very low temperatures. The results show the potential of this device to characterise samples of proteins and other molecules at levels of a few pL, and in a temperature range inaccessible to commercial EPR systems.
Title: “On-chip EPR spectrometry of metalloproteins using superconducting lumped element resonators”
Nanoscale 2025, doi:10.1039/d5nr03119b
4th December 2025
Abstract: We report electron paramagnetic resonance experiments performed on myoglobin hemeproteins using a chip hosting 6 superconducting lumped element resonators with resonance frequencies between 1.94 and 2.11 GHz. Successive layers of myoglobin were deposited onto the inductors of four of them using dip-pen nanolithography, a technique based on atomic force microscopy. A combination of atomic force and confocal microscopies estimated the number of protein molecules in each deposit, which ranges from 8.6 × 1011 (one dip-pen layer) to 3.33 × 1012 (four dip-pen layers). Two reference bulk samples were pipetted from the same solution onto the remaining two resonators. The microwave transmission of the device, measured at 11 mK, shows evidence of the coupling of protein spins to the photon excitations of all resonators. In particular, the resonance broadening measured as a function of magnetic field provides the spin resonance absorption spectrum. The analysis suggests that proteins tend to self-orient on the chip. It also allows estimating the single spin to single photon coupling strength, which is around 9 Hz. This high coupling value suggests that dip-pen nanolithography gives rise to a close to optimum interface between the molecules and the chip surface. The developed methodology combines an increase in sensitivity of at least three orders of magnitude with the ability to characterize multiple samples in a single experiment, opening the door to a highly sensitive multi-analyte detection technology.