The surprising properties of cold matter
The study of matter at very low temperatures has yielded surprising results. For example, superconductivity. The Dutch physicist Heike Kamerlingh Onnes succeeded in liquefying helium in 1908. A great achievement considering that helium boils at -269°C (helium has the lowest boiling temperature and at atmospheric pressure never freezes). Onnes used liquid helium to cool other substances, such as mercury, which solidifies at -38.89º C. When he measured its electrical resistance, he found, as expected, that the lower the temperature of the mercury, the lower its resistance. But when he reached -269º C he discovered that its electrical resistance became zero: Kamerlingh Onnes had just discovered superconductivity. Since then, different materials have been discovered which, below a certain critical temperature, offer no resistance to the passage of electricity: superconductors.
Superfluidity
Kamerlingh Onnes missed one of the most surprising properties of liquid helium. In 1938, Russian Peter Kapitsa and Canadians John Allen and Austin Misener found that below -271°C liquid helium became an excellent conductor of heat, 200 times better than copper. Not only that, but it had a viscosity less than one ten-thousandth of that of hydrogen gas: this is the phenomenon of superfluidity.
All liquids have an opposition to flow: it is viscosity, the product of friction between the molecules of the solid and those of the surface on which they slide. Some, like shampoo or honey, are very viscous. Others, such as water, are not so viscous. The same is true of liquid helium. However, below -271°C, its viscosity practically disappears and it becomes superfluid. This means that we can see how the helium literally rises up the walls of the vessel containing it and spills out. This fact has important technological applications, such as the location of micro-holes in ducts and pipes: superfluid helium can be easily squeezed through holes smaller than 2 ten-thousandths of a millimetre.
At the limit
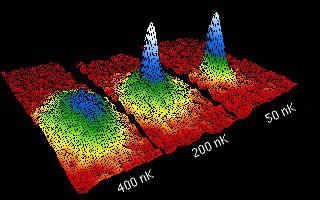
In this amazing world of very low temperature phenomena, a group at the Joint Institute for Laboratory Astrophysics (JILA) in Boulder, Colorado, culminated in 1995 a two-decade effort by scientists around the world to experimentally verify a prediction made almost 80 years ago by Albert Einstein and the Indian Satyendra Nath Bose. At normal temperatures, the atoms of a gas are distributed throughout the volume of the container that holds them. But at extremely low temperatures, of the order of a few billionths of a degree above absolute zero, the atoms behave as if they were a single superatom: this is the Bose-Einstein condensate, a new state of matter. The JILA group succeeded in cooling 2,000 rubidium atoms to below one hundred billionths of a degree absolute for 10 seconds, creating the first ever Bose-Einstein condensate. They received the Nobel Prize for it.