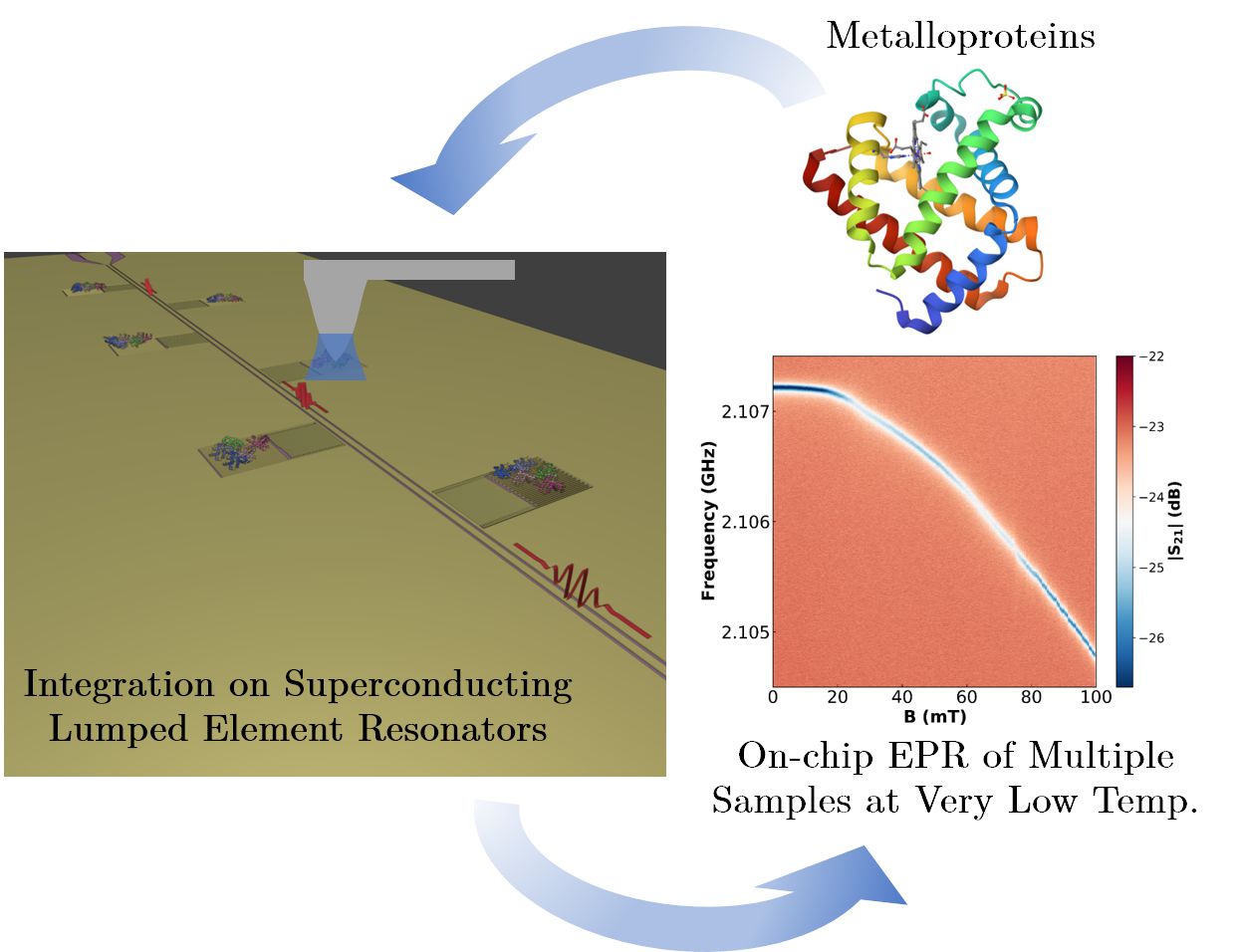
On-chip EPR spectrometry of metalloproteins using superconducting lumped element resonators
Investigadores del grupo Quantum Materials and Devices | Q-MAD e HYMAT del INMA, en estrecha colaboración con investigadores del Centro de Astrobiología (CAB, CSIC-INTA), han desarrollado una plataforma EPR en chip basada en resonadores LER superconductores para la caracterización múltiple de cantidades muy pequeñas de metaloproteínas. El sensor se ha verificado mediante la integración con nanolitografía de dip-pen (DPN) de cantidades controladas de moléculas de mioglobina. El valor del acoplamiento promedio espín-fotón es alto, de unos 9 Hz, lo que sugiere que el método de integración por DPN genera una interfaz casi óptima entre las moléculas y la superficie del chip. El sensor combina un aumento de la sensibilidad de las medidas de entre 3 y 4 órdenes de magnitud respecto a los equipos de EPR convencional con la habilidad de poder caracterizar múltiples muestras a la vez a muy bajas temperaturas. Los resultados muestran el potencial de este dispositivo para caracterizar muestras de proteínas y otras moléculas a nivel de unos pocos pL, y en una región de temperatura inaccesible a los sistemas comerciales de EPR.
Título: «On-chip EPR spectrometry of metalloproteins using superconducting lumped element resonators»
Nanoscale 2025, doi:10.1039/d5nr03119b
4th December 2025
Abstract: We report electron paramagnetic resonance experiments performed on myoglobin hemeproteins using a chip hosting 6 superconducting lumped element resonators with resonance frequencies between 1.94 and 2.11 GHz. Successive layers of myoglobin were deposited onto the inductors of four of them using dip-pen nanolithography, a technique based on atomic force microscopy. A combination of atomic force and confocal microscopies estimated the number of protein molecules in each deposit, which ranges from 8.6 × 1011 (one dip-pen layer) to 3.33 × 1012 (four dip-pen layers). Two reference bulk samples were pipetted from the same solution onto the remaining two resonators. The microwave transmission of the device, measured at 11 mK, shows evidence of the coupling of protein spins to the photon excitations of all resonators. In particular, the resonance broadening measured as a function of magnetic field provides the spin resonance absorption spectrum. The analysis suggests that proteins tend to self-orient on the chip. It also allows estimating the single spin to single photon coupling strength, which is around 9 Hz. This high coupling value suggests that dip-pen nanolithography gives rise to a close to optimum interface between the molecules and the chip surface. The developed methodology combines an increase in sensitivity of at least three orders of magnitude with the ability to characterize multiple samples in a single experiment, opening the door to a highly sensitive multi-analyte detection technology.